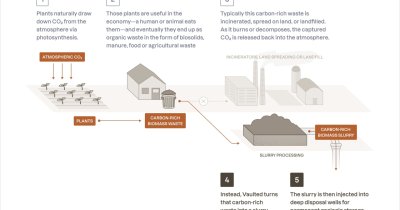
Phys writes that the findings were published in Nature Communications and the team of researchers showcased how formic acid can transform carbon emissions into substances used by the biochemical industry.
Besides carbon dioxide, energy is also needed to complete the process and it can be sourced from renewable sources, such as solar panels.
Maren Nattermann, first author of the study, says that "formic acid is a very promising carbon source. But converting it to formaldehyde in the test tube is quite energy-intensive", which is why she and her team is looking at ways to make the product using less power, as this would help reduce the cost, while also enable using more energy to produce more substance.
Optimizing the enzymes used in the process is also a crucial way by which cost can be reduced and this was already done, as the researchers collaborated with Festo, a Germany-based company that was able to develop around 4.000 combinations. According to Maren, this resulted in a production four times more efficient.
One of the long-term goals with this project is to turn carbon dioxide emissions into useful products, such as biodiesel and even insulin.
 Mihai - Cristian Ioniță
Mihai - Cristian Ioniță










Any thoughts?